What Is The Difference Between N And M In Chemistry . both m and m are units of the concentration of a chemical solution. converting from molarity to normality. Relation between normality and molarity. for some chemical solutions, normality and molarity are equivalent or n=m. Differences between normality and molarity. A capital m signifies solutions labelled with molar. molarity is the number of moles of a substance per litre of solution, also known as molar concentration. Here are some key differences between normality and molarity. The lowercase m indicates molality, which is calculated using moles of. the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. How to convert molarity to. ⇒ n = m × number of equivalents. You can convert from molarity (m) to normality (n) using the following equation:
from www.doubtnut.com
both m and m are units of the concentration of a chemical solution. converting from molarity to normality. Here are some key differences between normality and molarity. You can convert from molarity (m) to normality (n) using the following equation: molarity is the number of moles of a substance per litre of solution, also known as molar concentration. The lowercase m indicates molality, which is calculated using moles of. the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. ⇒ n = m × number of equivalents. How to convert molarity to. A capital m signifies solutions labelled with molar.
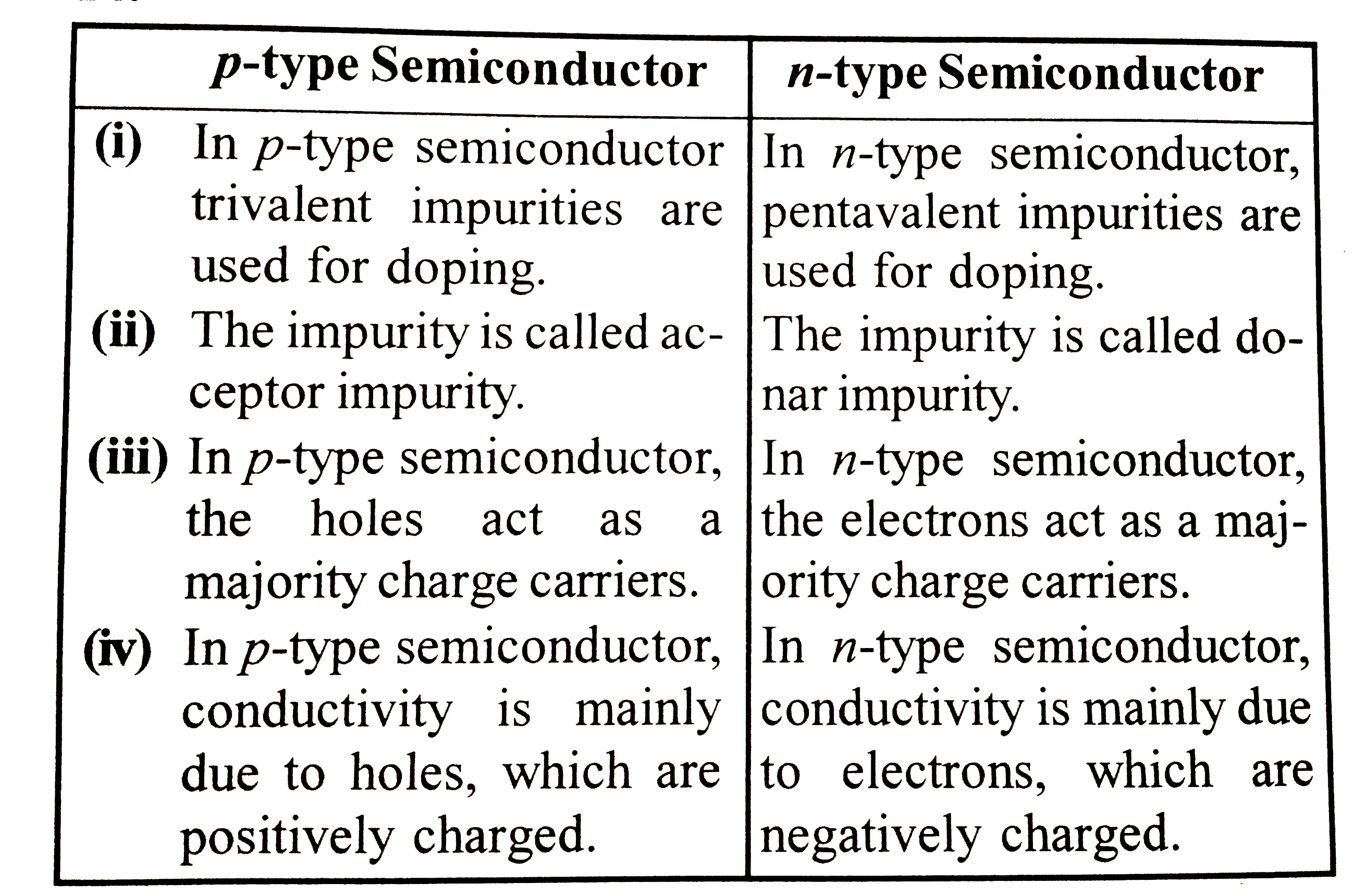
Distinguish between p type and n type semiconductors
What Is The Difference Between N And M In Chemistry A capital m signifies solutions labelled with molar. The lowercase m indicates molality, which is calculated using moles of. A capital m signifies solutions labelled with molar. both m and m are units of the concentration of a chemical solution. molarity is the number of moles of a substance per litre of solution, also known as molar concentration. Relation between normality and molarity. ⇒ n = m × number of equivalents. the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. for some chemical solutions, normality and molarity are equivalent or n=m. You can convert from molarity (m) to normality (n) using the following equation: Differences between normality and molarity. converting from molarity to normality. How to convert molarity to. Here are some key differences between normality and molarity.
From www.pinterest.ch
The following Venn diagram shows the similarities and differences What Is The Difference Between N And M In Chemistry Relation between normality and molarity. A capital m signifies solutions labelled with molar. Differences between normality and molarity. both m and m are units of the concentration of a chemical solution. The lowercase m indicates molality, which is calculated using moles of. How to convert molarity to. molarity is the number of moles of a substance per litre. What Is The Difference Between N And M In Chemistry.
From www.pinterest.com.au
Organic Chemistry Nomenclature Organic chemistry, Study chemistry What Is The Difference Between N And M In Chemistry You can convert from molarity (m) to normality (n) using the following equation: converting from molarity to normality. both m and m are units of the concentration of a chemical solution. ⇒ n = m × number of equivalents. molarity is the number of moles of a substance per litre of solution, also known as molar. What Is The Difference Between N And M In Chemistry.
From www.youtube.com
How To Calculate Normality & Equivalent Weight For Acid Base Reactions What Is The Difference Between N And M In Chemistry Relation between normality and molarity. Here are some key differences between normality and molarity. the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. both m and m are units of the concentration of a chemical solution. A capital m signifies solutions labelled with. What Is The Difference Between N And M In Chemistry.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce What Is The Difference Between N And M In Chemistry for some chemical solutions, normality and molarity are equivalent or n=m. both m and m are units of the concentration of a chemical solution. You can convert from molarity (m) to normality (n) using the following equation: How to convert molarity to. molarity is the number of moles of a substance per litre of solution, also known. What Is The Difference Between N And M In Chemistry.
From www.pinterest.com
How to Calculate Normality of a Solution Chemistry lessons, Chemistry What Is The Difference Between N And M In Chemistry the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. both m and m are units of the concentration of a chemical solution. You can convert from molarity (m) to normality (n) using the following equation: Relation between normality and molarity. molarity is. What Is The Difference Between N And M In Chemistry.
From telgurus.co.uk
How to calculate moles in chemistry? Chemistry questionnaire What Is The Difference Between N And M In Chemistry Here are some key differences between normality and molarity. for some chemical solutions, normality and molarity are equivalent or n=m. converting from molarity to normality. The lowercase m indicates molality, which is calculated using moles of. A capital m signifies solutions labelled with molar. You can convert from molarity (m) to normality (n) using the following equation: . What Is The Difference Between N And M In Chemistry.
From slidesharenow.blogspot.com
Difference Between Compound And Molecule In Chemistry slideshare What Is The Difference Between N And M In Chemistry Here are some key differences between normality and molarity. the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. The lowercase m indicates molality, which is calculated using moles of. ⇒ n = m × number of equivalents. Differences between normality and molarity. Relation. What Is The Difference Between N And M In Chemistry.
From www.pinterest.com
Naming Alkanes by IUPAC nomenclature Rules Practice Problems What Is The Difference Between N And M In Chemistry converting from molarity to normality. the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. How to convert molarity to. You can convert from molarity (m) to normality (n) using the following equation: molarity is the number of moles of a substance per. What Is The Difference Between N And M In Chemistry.
From genius.com
Chemistry Genius Units of Measurement Genius What Is The Difference Between N And M In Chemistry You can convert from molarity (m) to normality (n) using the following equation: A capital m signifies solutions labelled with molar. both m and m are units of the concentration of a chemical solution. converting from molarity to normality. Differences between normality and molarity. molarity is the number of moles of a substance per litre of solution,. What Is The Difference Between N And M In Chemistry.
From mavink.com
Chemistry 1 Conversion Chart What Is The Difference Between N And M In Chemistry The lowercase m indicates molality, which is calculated using moles of. A capital m signifies solutions labelled with molar. You can convert from molarity (m) to normality (n) using the following equation: both m and m are units of the concentration of a chemical solution. converting from molarity to normality. How to convert molarity to. Differences between normality. What Is The Difference Between N And M In Chemistry.
From dxoofepvw.blob.core.windows.net
Network Definition Chemistry at Ronald Hou blog What Is The Difference Between N And M In Chemistry both m and m are units of the concentration of a chemical solution. ⇒ n = m × number of equivalents. A capital m signifies solutions labelled with molar. for some chemical solutions, normality and molarity are equivalent or n=m. The lowercase m indicates molality, which is calculated using moles of. the relation between normality and. What Is The Difference Between N And M In Chemistry.
From pediaa.com
Difference Between Physical and Chemical Change Definition, Examples What Is The Difference Between N And M In Chemistry the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. Here are some key differences between normality and molarity. molarity is the number of moles of a substance per litre of solution, also known as molar concentration. both m and m are units. What Is The Difference Between N And M In Chemistry.
From www.doubtnut.com
Distinguish between p type and n type semiconductors What Is The Difference Between N And M In Chemistry ⇒ n = m × number of equivalents. molarity is the number of moles of a substance per litre of solution, also known as molar concentration. How to convert molarity to. both m and m are units of the concentration of a chemical solution. for some chemical solutions, normality and molarity are equivalent or n=m. Here. What Is The Difference Between N And M In Chemistry.
From www.youtube.com
What is the Difference between Shell and Subshell Chemistry Class 9 What Is The Difference Between N And M In Chemistry You can convert from molarity (m) to normality (n) using the following equation: Here are some key differences between normality and molarity. A capital m signifies solutions labelled with molar. Differences between normality and molarity. ⇒ n = m × number of equivalents. the relation between normality and molarity is n = m x n where n refers. What Is The Difference Between N And M In Chemistry.
From docentejohngiraldo2023.blogspot.com
Doc. John Alexander Giraldo M. febrero 2023 What Is The Difference Between N And M In Chemistry The lowercase m indicates molality, which is calculated using moles of. How to convert molarity to. ⇒ n = m × number of equivalents. Differences between normality and molarity. converting from molarity to normality. both m and m are units of the concentration of a chemical solution. Relation between normality and molarity. molarity is the number. What Is The Difference Between N And M In Chemistry.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples What Is The Difference Between N And M In Chemistry the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. molarity is the number of moles of a substance per litre of solution, also known as molar concentration. Here are some key differences between normality and molarity. A capital m signifies solutions labelled with. What Is The Difference Between N And M In Chemistry.
From learningschooljfreyre8d.z22.web.core.windows.net
Organic Vs Compounds Examples What Is The Difference Between N And M In Chemistry Differences between normality and molarity. Here are some key differences between normality and molarity. for some chemical solutions, normality and molarity are equivalent or n=m. A capital m signifies solutions labelled with molar. both m and m are units of the concentration of a chemical solution. You can convert from molarity (m) to normality (n) using the following. What Is The Difference Between N And M In Chemistry.
From pediaa.com
Difference Between Organic and Chemistry Definition What Is The Difference Between N And M In Chemistry the relation between normality and molarity is n = m x n where n refers to normality, m is molarity, and n denotes the. for some chemical solutions, normality and molarity are equivalent or n=m. Relation between normality and molarity. ⇒ n = m × number of equivalents. converting from molarity to normality. How to convert. What Is The Difference Between N And M In Chemistry.